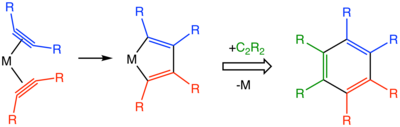
Alkyne trimerisation
|
Read other articles:

Norway-related events during the year of 1958 This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: 1958 in Norway – news · newspapers · books · scholar · JSTOR (October 2022) (Learn how and when to remove this template message) ← 1957 1956 1955 1958 in Norway → 1959 1960 1961 Centuries: 18th 19th 20th...

Republik PortugisRepública Portuguesa1910–1926 Bendera Lambang Lagu kebangsaan: A Portuguesacode: pt is deprecated (Portugis)The PortuguesenoiconIbu kotaLisbonBahasa yang umum digunakanBahasa PortugisPemerintahanRepublik parlementer partai dominanPresiden • 1911–1915 Manuel de Arriaga (pertama)• 1925–1926 Bernardino Machado (terakhir) Perdana Menteri • 1911 João Pinheiro Chagas (pertama)• 1925–1926 António Maria da Silva (...

Kunsthistorisches MuseumDidirikan1871-1891LokasiWina, AustriaWisatawan559.150 (2010)[1]Situs webhttp://www.khm.at Kunsthistorisches Museum (bahasa Indonesia: Museum Sejarah Seni) adalah sebuah museum seni yang terletak di kota Wina, Austria. Museum ini terletak di sebuah istana di Ringstraße dan merupakan museum seni terbesar di Austria. Museum ini dibuka ada tahun 1891 oleh Kaisar Franz Joseph I dari Austria-Hungaria bersamaan dengan Museum Sejarah Alam. Kedua museum ini memiliki ke...

تاريخ تركمانستانمعلومات عامةالمنطقة تركمانستان التأثيراتفرع من اهمية اسيا الوسطى استراتجياً تعديل - تعديل مصدري - تعديل ويكي بيانات يعود تاريخ تركمانستان إلى عصور غابرة في القدم، حيث يرجع أول استيطان للإنسان فيها إلى العصر الحجري القديم، والبقايا الأثرية المكتشفة في ال�...

Penjaga gawang Wales Danny Ward menangkap bola dalam laga melawan Swiss. Pertandingan Grup A Kejuaraan Eropa UEFA 2020 berlangsung pada tanggal 11 hingga 20 Juni 2021 di Stadion Olimpiade, Baku dan Stadio Olimpico, Roma.[1] Grup ini terdiri dari Turki, tuan rumah Italia, Wales, dan Swiss. Tim peserta Posisi undian Tim Pot Metodekualifikasi Tanggallolos Penampilan diputaran final Penampilanterakhir Pencapaianterbaik Peringkat KualifikasiNovember 2019[nb 1] Peringkat FIFAMei 202...

Species of bird Red-shouldered hawk A red-shouldered hawk near Blue Cypress Lake, Florida Conservation status Least Concern (IUCN 3.1)[1] Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Aves Order: Accipitriformes Family: Accipitridae Genus: Buteo Species: B. lineatus Binomial name Buteo lineatus(Gmelin, 1788) Range of B. lineatus Breeding range Year-round range Wintering range The red-shouldered hawk (B...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (مايو 2021) التمييز العصبي على أساس الجنس هو تحيز مزعوم في علم أعصاب الاختلافات الجنسية لتعزيز القوالب النمطية الضارة بين الجنسين. صاغت الباحثة النسوية كورديليا فاين...

Campeonato Brasileiro de Futebol Americano de 2017 Dados Participantes 70 Organização Liga BFALNFALINEFA Período 1 de julho - 10 de dezembro ◄◄2016 2018►► O Campeonato Brasileiro de Futebol Americano de 2017 foi uma competição organizada pelas ligas de clubes Liga Brasil Futebol Americano (Liga BFA), Liga Nacional de Futebol Americano (LNFA) e Liga Nordestina de Futebol Americano (LINEFA), chanceladas pela Confederação Brasileira de Futebol Americano (CBFA). A Liga BFA organizo...

Cirrhilabrus wakanda TaksonomiKerajaanAnimaliaFilumChordataKelasActinopteriOrdoPerciformesFamiliLabridaeGenusCirrhilabrusSpesiesCirrhilabrus wakanda Yi-Kai Tea, Pinheiro, Bart Shepherd dan Rocha, 2019 Tata namaDinamakan berdasarkanWakanda lbs Cirrhilabrus wakanda[1][2] adalah sebuah spesies ikan dari Samudra Hindia Barat. Spesies tersebut mengambil nama dari negara berdaulat fiksi, Wakanda dari Marvel Comics[3] dan Marvel Cinematic Universe. Referensi ^ Weiss, Josh (20...

Para otros usos de este término, véase Panamá (desambiguación). República de PanamáBandera Escudo Lema: Pro mundi beneficio(en latín: «Para beneficio del mundo») Himno: «Himno Nacional de Panamá» ¿Problemas al reproducir este archivo? Capital(y ciudad más poblada) Ciudad de Panamá 9°00′N 79°30′O / 9, -79.5 Idioma oficial EspañolGentilicio Panameño, -ñaForma de gobierno República presidencialista • Presidente Laurentino Cortizo R...

Este artículo o sección necesita referencias que aparezcan en una publicación acreditada.Este aviso fue puesto el 13 de julio de 2018. Imagen de la NASA del cohete acelerador sólido (derecha) siendo adjuntado al Delta II (azul). Se pueden ver dos cohetes (blanco) ya agregados. Los Cohetes Aceleradores Sólidos (SRB, Solid Rocket Boosters, o SRM, para denominar a los motores en sí) son usados para proporcionar empuje principal a los lanzamientos de naves espaciales desde la plataforma de ...

Peta bahasa Serbia-Kroasia terbagi dalam dialek-dialek besarnya. Konferensi pers tentang Deklarasi tentang Bahasa Umum Deklarasi tentang Bahasa Umum (bahasa Serbo-Kroasia: Deklaracija o zajedničkom jeziku / Декларација о заједничком језику) dikeluarkan pada 2017 oleh sekelompok intelektual dan organisasi non-pemerintahan dari Kroasia, Bosnia dan Herzegovina, Montenegro, dan Serbia yang berkarya di bawah naungan dari sebuah proyek yang disebut Bahasa dan Nasio...

Umayyad caliph from 717 to 720 Umar IIعمر بن عبد العزيز Amir al-Mu'minin Khalifat Allah Gold dinar of Umar II, minted in Damascus, 719/208th Caliph of the Umayyad CaliphateReign22 September 717/99 AH – 4 February 720 CE/101 AHPredecessorSulayman ibn Abd al-MalikSuccessorYazid IIBorn2 November 680/61 AHMedina, Arabia, Umayyad CaliphateDiedc. 5 February 720 CE/101 AH (aged 39)Dayr Sim'an, Syria, Umayyad CaliphateBurialDayr Sim'an, Syria, Umayyad CaliphateWife Fatima bint A...

American writer and actor Robert BenchleyBenchley photographed for Vanity FairBornRobert Charles Benchley(1889-09-15)September 15, 1889Worcester, Massachusetts, U.S.DiedNovember 21, 1945(1945-11-21) (aged 56)New York City, U.S.OccupationWriter, critic, actor, film directorGenreDeadpan, Parody, Surreal humourYears active1910s-1945Spouse Gertrude Darling (m. 1914)Children2, including NathanielRelativesPeter Benchley and Nat Benchley (grandsons) Robert Ch...

Tour TotalInformasi umumLokasiLa Défense(Courbevoie)TinggiAtap187m (689 (kaki)Data teknisJumlah lantai48Luas lantai130,000 m² (1.4 juta kaki²)Desain dan konstruksiArsitekWZMH Architects, Roger Saubot Tour Total (sebelumnya dikenal sebagai Tour Elf sejak 1985 hingga 1999, dan Tour TotalFinalElf sejak 1999 hingga 2003) merupakan sebuah pencakar langit perkantoran yang terletak di La Défense, Courbevoie, distrik bisnis besar di barat dan dekat kota Paris, Prancis dan sekarang merupakan kanto...

«URL» redirige aquí. Para otras acepciones, véase URL (desambiguación). Este diagrama de Euler muestra que un identificador de recursos uniforme (URI) es o bien un localizador uniforme de recursos (siglas en inglés URL), un nombre de recurso uniforme (URN, siglas en inglés), o ambos a la vez. Un LRU o localizador de recursos uniforme (más conocido por las siglas URL, del inglés Uniform Resource Locator)[1] es un identificador de recursos uniforme (Uniform Resource Identifier, ...

Nina MenshikovaBorn(1928-08-08)8 August 1928MoscowDied26 December 2007(2007-12-26) (aged 79)MoscowNationalityRussianOccupationActress Nina Yevgenyevna Menshikova (Russian: Ни́на Евге́ньевна Ме́ньшикова; 8 August 1928 – 26 December 2007)[1] was a Soviet actress. She was the wife of Stanislav Rostotsky and the mother of Andrei Rostotsky. Nina Menshikova was awarded the title of People's Artist of the RSFSR in 1977 and also have received USSR State P...

Logging industry work site Lumberjacks in front of logging camp building A logging camp (or lumber camp) is a transitory work site used in the logging industry. Before the second half of the 20th century, these camps were the primary place where lumberjacks would live and work to fell trees in a particular area. Many place names (e.g. Bockman Lumber Camp, Whitestone Logging Camp, Camp Douglas) are legacies of old logging camps. Camps were often placed next to river tributaries so that the win...

هيرمان فون بالان (بالألمانية: Hermann Ludwig von Balan) معلومات شخصية الميلاد 7 مارس 1812(1812-03-07)برلين الوفاة 16 مارس 1874 (62 سنة)بروكسل مناصب الحياة العملية المهنة دبلوماسي، وسياسي تعديل مصدري - تعديل هيرمان فون بالان Hermann Ludwig von Balan (ولد في مارس 1812 في برلين – ومات في �...

Die Grünen Kärnten Logo Basisdaten Landessprecher: Landtagsmandate 0/36 Landessprecherin: Olga Voglauer[1] OrganisatorischerGeschäftsführer: Jonathan Seriatz Hauptsitz: Bahnhofstraße 38a9020 Klagenfurt am Wörthersee Website: Die Grünen Kärnten Olga Voglauer 2023 Die Grünen Klagenfurt 2023 Die Grünen Kärnten bzw. Die Grünen – Die Grüne Alternative Kärnten ist die autonome Landesorganisation der österreichischen Partei Die Grünen – Die Grüne Alternative in Kärnten....