Conservation of mass
|
Read other articles:

American college football season 2015 Montana Grizzlies footballFCS Playoffs Second Round, L 6–37 vs. North Dakota StateConferenceBig Sky ConferenceRankingSTATSNo. 14FCS CoachesNo. 14Record8–5 (6–2 Big Sky)Head coachBob Stitt (1st season)Defensive coordinatorTy Gregorak (4th season)Home stadiumWashington–Grizzly Stadium(Capacity: 25,217)Seasons← 20142016 → 2015 Big Sky Conference football standings vte Conf Overall Team W L ...

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (نوفمبر 2018) إيلين إيسيل (بالإنجليزية: Eileen Essell) معلومات شخصية الميلاد 8 أكتوبر 1922 لندن الوفاة 15 فبراير 2015 (92 سنة) لندن سبب الوفاة مرض مواطنة المملك

此條目疑似由大量爱好者内容组成。 (2023年8月1日)維基百科不是不經篩選的資訊收集處。請幫助改進這個條目,使用中立的語氣(而不是愛好者或媒體報道的語氣),移除瑣碎的軼事與未經證實的評論、不合適的列表和链接收集等。如條目內有愛好者可能感興趣而不符維基百科收錄標準的內容,可考慮將該等內容移至其他專門描寫來自深淵 (漫畫)的百科或網站,或在不存在相

American architect (1853–1911) For the merchant and politician in New Brunswick, see Bradford Gilbert (politician). Bradford Lee GilbertBorn(1853-03-24)March 24, 1853Watertown, New York, USDiedSeptember 1, 1911(1911-09-01) (aged 58)Accord, New York, U.S.OccupationArchitectSpouse(s)Cora RathboneMaria Fahy McAuleyAwardsWorld's Columbian Exhibition – Gold MedalCotton States and International Exposition – Gold MedalPracticeBradford L. Gilbert1 Broadway, 50 BroadwayNew York City, New Yo...

Victor Lindelöf Lindelöf con il Manchester United nel 2017 Nazionalità Svezia Altezza 187 cm Peso 82 kg Calcio Ruolo Difensore Squadra Manchester Utd Carriera Giovanili ???? IK Franke???? Västerås IK2007-2009 Västerås SK2012-2013 Benfica Squadre di club1 2009-2012 Västerås SK50 (0)2012-2016 Benfica B96 (4)2013-2017 Benfica48 (2)2017- Manchester Utd166 (4) Nazionale 2009-2011 Svezia U-175 (0)2012-2013 Svezia U-1917 (0)2014-2015 Svezia U-2...

كوم أبو حجر - قرية مصرية - تقسيم إداري البلد مصر المحافظة محافظة أسيوط المركز صدفا المسؤولون السكان التعداد السكاني 3959 نسمة (إحصاء 2006) معلومات أخرى التوقيت ت ع م+02:00 تعديل مصدري - تعديل قرية كوم أبو حجر هي إحدى القرى التابعة لمركز صدفا في محافظة أسيوط في ج

Faye KusairiLahirDayangku Faratiwan Adnil binti Awang Kusairi19 Juni 1987 (umur 36)Miri, Sarawak, MalaysiaPekerjaanAktris, penyanyi, modelTahun aktif2013–kiniTinggi1.545 cm (50 ft 8 in)Suami/istriAzmi Hatta (berkahwin 2017)Anak1 Dayangku Faratiwan Adnil binti Awang Kusairi (lahir 19 Juni 1987) atau lebih dikenal sebagai Faye Kusairi (Jawi: فاياي كوسايري) adalah seorang aktris dan model wanita Malaysia dari Miri, Sarawak Negeri Bumi Kenyalang. Ia mulai di...

1993 novel by John Banville Ghosts First editionAuthorJohn BanvilleCountryIrelandLanguageEnglishPublisherSecker and WarburgPublication date1993Media typePrint (Hardcover & Paperback)Pages224ISBN0-436-19991-2Preceded byThe Book of Evidence Followed byAthena Ghosts is a 1993 novel by John Banville. It was his first novel since 1989's The Book of Evidence, which was shortlisted for the Booker Prize. The second in what Banville described as a triptych, to make an inv...

OmniROMTangkapan layar dari OmniROM 6.0.1Perusahaan / pengembangKomunitas OmniROMDiprogram dalamC (inti), C++ (beberapa pustaka pihak ketiga), Java (UI)KeluargaSistem operasi benam (Linux/Android)Status terkiniAktifModel sumberSumber terbukaRilis stabil terkini13.0[1]Target pemasaranPengganti perangkat tegar untuk peranti bergerak AndroidManajer paketAPKKernel typeMonolitik (kernel Linux)LisensiLisensi perangkat lunak bebas:Lisensi Apache 2.0 dan GNU GPLv2Situs web resmiomnirom.o...

Gosong pasir pasang-surut yang menghubungkan Pulau Waya dan Wayasewa di Kepulauan Yasawa, Fiji. Gumuk pasir di Frisian Utara Laut Wadden (Jerman) Gosong pasir, atau gosong saja, adalah bentukan daratan yang terkurung atau menjorok pada suatu perairan. Gosong biasa terbentuk dari pasir, geluh, atau kerikil. Bentukan geografi ini terjadi akibat adanya aliran dangkal dan sempit sehingga memungkinkan pengendapan material ringan dan mengarah pada pendangkalan tubuh air. Gosong dapat terbentuk di l...

У Вікіпедії є статті про інші значення цього терміна: Церква Покрови Пресвятої Богородиці. Шаблон:Визначна пам'ятка: невідомі параметри: завершення будівництваЦерква Покрови Пресвятої Богородиці (Кровинка) Тип церкваКраїна Україна : ISO3166-1 alpha-3:UKR; ISO3166-1 цифровий...

Bù Đốp Camp(Bù Đốp Airfield) Bù Đốp District, Bình Phước Province in VietnamBù Đốp Camp, 23 September 1967Bù Đốp CampShown within VietnamCoordinates12°01′07″N 106°48′52″E / 12.01861°N 106.81444°E / 12.01861; 106.81444TypeArmy baseSite informationOperatorArmy of the Republic of Vietnam (ARVN)United States Army (U.S. Army)ConditionAbandonedSite historyBuilt1963 (1963)In use1963-1974 (1974)Battles/warsVietnam W...

الجهمية الدين إسلام المؤسس الجهم بن صفوان مَنشأ الكوفة بالعراق الأركان خلق القرآن - الإرجاء - تعطيل الصفات - الجبر الامتداد خراسان - العراق ( حالياً لا يوجد لها أتباع ) تعديل مصدري - تعديل الجهميةُ أو المُعَطِّلَةُ هي فرقةٌ كلاميَّة تنتسب إلى الإسلام. وهي إحدى فرق غلاة الم...

Science during the 16th-19th century Table of astronomy, from the 1728 Cyclopaedia The history of science during the Age of Enlightenment traces developments in science and technology during the Age of Reason, when Enlightenment ideas and ideals were being disseminated across Europe and North America. Generally, the period spans from the final days of the 16th and 17th-century Scientific Revolution until roughly the 19th century, after the French Revolution (1789) and the Napoleonic era (1799...

2008 live album by Cecil TaylorCT: The Dance ProjectLive album by Cecil TaylorReleased2008RecordedJuly 8, 1990VenueAkademie der Kunste, BerlinGenreJazzLength39:16LabelFMPProducerJost Gebers CT: The Dance Project is a live album by Cecil Taylor recorded during the Summer Music concert series at the Akademie der Kunste, Berlin on July 8, 1990, and released in 2008 by FMP. The album documents a multimedia event that featured Taylor, bassist William Parker, percussionist Masashi Harada, a...

1990 American filmMob BossVHS coverDirected byFred Olen RayScreenplay byT. L. LankfordProduced byMark AminJeffrey B. MallianFred Olen RayGrant Austin WaldmanStarringEddie DeezenMorgan FairchildWilliam HickeyIrwin KeyesCinematographyGary GraverEdited byChristopher RothMusic byChuck CirinoProductioncompanyAmerican International PicturesRelease date September 16, 1990 (1990-09-16) Running time93 minutesCountryUnited StatesLanguageEnglish Mob Boss is a 1990 direct-to-video Mafia-th...

Государство Бруней-Даруссаламмалайск. Negara Brunei Darussalamджави: نڬارا بروني دارالسلام Флаг Эмблема Девиз: «Brunei Darussalam»(малайский: Бруней — обитель мира); второй девиз: «Всегда служить под предводительством Бога» Гимн: «Allah, Peliharakan Sultan» Бруней на карте региона Дата независимос�...
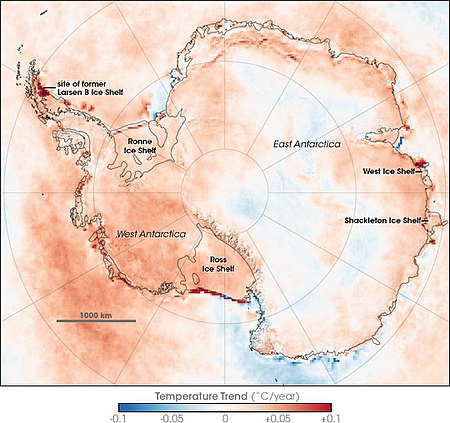
Tendencias de temperatura de la superficie antártica entre 1981 y 2007, basadas en observaciones infrarrojas térmicas realizadas por una serie de sensores satelitales NOAA. Las tendencias de la temperatura de la superficie no reflejan necesariamente las tendencias de la temperatura del aire. Los efectos del calentamiento global en la Antártida se deben al aumento de las temperaturas, con el consiguiente aumento del derretimiento de la nieve y la pérdida de hielo.[1] Un artículo pu...

Bagus Suryadi TayoWaasrena Kasad Bid. Manajemen dan Reformasi BirokrasiPetahanaMulai menjabat 21 Januari 2022PendahuluMukhlisWakil Inspektur KostradMasa jabatan28 Desember 2020 – 21 Januari 2022PendahuluSidhi PurnomoPenggantiHenra Hari Sutaryo Informasi pribadiLahir23 Agustus 1971 (umur 52)Bandung, Jawa BaratSuami/istriNy. Anne SarvitriHubunganTayo Tarmadi (ayah)Alma materAkademi Militer (1993)Karier militerPihak IndonesiaDinas/cabang TNI Angkatan DaratMasa dinas...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Scent of a Woman seri televisi – berita · surat kabar · buku · cendekiawan · JSTOR Scent of a WomanBerkas:Scent of a Woman-poster.jpgGenreRomansaDramaSutradaraPark Hyung-kiPemeranKim Sun-aLee Dong-w...















