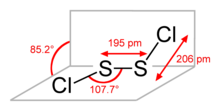
Disulfur dichloride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Fridtjuv Berg (1851–1916) Gatskyltar från Övre Slottsgatan i Uppsala: en med en äldre stavning (”Öfre”) norr om S:t Olofsgatan, och en med en nyare stavning (”Övre”) söder därom. Burk med vitpeppar med gammalstavningen hvit. Stavningsreformen som genomdrevs 1906 förenklade stavningen av v-ljudet och t-ljudet i svenska språket. Stavningsreformen genomfördes på initiativ av den svenske ecklesiastikministern Fridtjuv Berg i den Staafska ministären efter 1905 års val. Refor...

The article is about the list of state highways in the Indian state of Andhra Pradesh. The state has a total of 14,722 km (9,148 mi) of State Highways and they account for 29% of the total roads in the state. SH 31 is the longest State Highway in Andhra Pradesh. The new state highways are recently added and new numbering system is given for state highways to improve infrastructure and connectivity.[1][2] S.No State Highway No. Route Districts connecting 1 NH167AG Pid...

BroccoflowerGreen cauliflowerSpesiesBrassica oleraceaKelompok budidayaBotrytis cultivar group Kembang kol hijau atau kembang brokoli adalah salah satu dari dua tanaman yang dapat dimakan dari spesies Brassica oleracea dengan kepala berwarna hijau muda. Bagian yang dapat dimakan adalah kepala bunga yang belum matang (perbungaan) dari tanaman. Brokoli dan kembang kol adalah kultivar yang berbeda dari spesies yang sama, dan karena itu keduanya dapat disilangkan baik dengan penyerbukan tangan ata...

كأس روسيا لكرة القدم 1992–93 تفاصيل الموسم كأس روسيا لكرة القدم النسخة 1 البلد روسيا التاريخ بداية:2 مايو 1992 نهاية:13 يونيو 1993 المنظم اتحاد روسيا لكرة القدم البطل توربيدو موسكو مباريات ملعوبة 512 عدد المشاركين 187 الكأس السوفيتي 1991–92 كأس روسيا لكر

Великобілозерський район адміністративно-територіальна одиниця Герб Прапор Колишній район на карті Основні дані Країна: Україна Область: Код КОАТУУ: 2321100000 Утворений: 3 лютого 1993 Ліквідований: 17 липня 2020 Населення: ▼ 7575 (01.11.2020) Площа: 470 км² Густота: 16.12 осіб/км² Тел. ко

Corona heráldica de un Príncipe de sangre. Luis IX de Francia o San Luis (1214-1270), algunos de sus descendientes fueron denominados desde el siglo XV príncipes de sangre. Luis Antonio de Borbón-Condé, duque de Enghien (1772-1804). Último descendiente agnático de la Casa de Condé. Murió fusilado por orden de Napoleón. Luis Francisco II de Borbón-Conti (1734-1814). Último representante de su linaje, murió sin hijos exiliado en España. La denominación príncipe de sangre er...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Plaza Pekalongan – berita · surat kabar · buku · cendekiawan · JSTOR Plaza PekalonganLokasiPekalonganAlamatJL. Nusantara No. 5, Pekalongan - Central Java Plaza PekalonganTanggal dibuka1994PengembangPT. W...

Romanian footballer and manager Not to be confused with Ion Marin. Marin Ion Personal informationDate of birth (1955-03-25) 25 March 1955 (age 68)Place of birth Ciorogârla, RomaniaHeight 1.87 m (6 ft 2 in)Position(s) DefenderYouth career1971–1972 Rapid București1972–1973 Dinamo BucureștiSenior career*Years Team Apps (Gls)1973–1984 Dinamo București 279 (4)1985–1986 Bihor Oradea 28 (1)1986 Victoria București 13 (0)1986–1987 Rapid București 26 (1)Total 346 (6)...

1977 live album by AlmanacAlmanacLive album by AlmanacReleased1977Recorded1967VenueColumbia University, New York CityGenreJazzLength32:57LabelImprovising ArtistsIAI 37.38.51ProducerMike Nock Almanac is a live album by the jazz ensemble of the same name, featuring pianist Mike Nock, saxophonist and flutist Bennie Maupin, double bassist Cecil McBee, and drummer Eddie Marshall. The group's sole release, it was recorded in 1967 at Columbia University in New York City, and was issued in 19...

Sporting event delegationUkraine at theUniversiadeIOC codeUKRNational federationUkrainian University Sports AssociationWebsitestudsport.com.uaMedalsRanked 8th Gold 216 Silver 229 Bronze 222 Total 667 Summer appearances 1993 1995 1997 1999 2001 2003 2005 2007 2009 2011 2013 2015 2017 2019 2021 Winter appearances 1993 1995 1997 1999 2001 2003 2005 2007 2009 2011 2013 2015 2017 2019 2023 Ukraine has participated in all Summer and Winter Universiades since the dissolution of the Soviet Union in 1...

German astronomer Richard Schorr Richard Reinhard Emil Schorr (20 August 1867, Kassel – 21 September 1951, Badgastein, Salzburg), was a German astronomer. Biography From 1889 to 1891, Schorr worked as an assistant editor of Astronomische Nachrichten, at the observatory at Kiel.[1] In 1892 Schorr became observer (observator) at the Hamburger Sternwarte (Hamburg Observatory) Schorr was the director of the Hamburger Sternwarte (Hamburg Observatory). The former director George Rümker h...

1996 single by Jay-Z Dead PresidentsSingle by Jay-Zfrom the album Reasonable Doubt ReleasedFebruary 20, 1996Recorded1995StudioD&D Studios (New York City)GenreMafioso rap[1]Length3:28 (Original)4:27 (Dead Presidents II)LabelRoc-A-FellaPrioritySongwriter(s) Shawn Carter David Willis Producer(s)SkiJay-Z singles chronology In My Lifetime (1995) Dead Presidents (1996) Ain't No Nigga (1996) Music videoDead Presidents on YouTube Dead Presidents is a 1996 song by American rapper Jay-Z. It...

Manual stimulation of a penis by a sex partner Johann Nepomuk Geiger, erotic watercolor, 1840. A woman giving a man a handjob. A handjob (also spelled hand job) is a manual sex act involving a person stimulating the penis or scrotum of another by using the hand.[1] This is done to induce an erection for sexual pleasure, sexual arousal and may result in orgasm and ejaculation.[2] A handjob can be performed as either foreplay or as non-penetrative sex. It is analogous to fingeri...

Czech ultralight aircraft Stream TL-Ultralight Stream at AERO Friedrichshafen 2018 Role Ultralight aircraftType of aircraft National origin Czech Republic Manufacturer TL-Ultralight Introduction 2013 Status In production (2017) The TL-Ultralight Stream is a Czech ultralight aircraft, designed and produced by TL-Ultralight of Hradec Králové, introduced at the AERO Friedrichshafen show in 2013.[1] Design and development The Stream is the company's fastest design to date. It was design...

South Korean actress In this Korean name, the family name is Choi. Choi Ja-hyeBorn (1981-07-26) July 26, 1981 (age 42)Seoul, South KoreaEducationSeoul Institute of the Arts - Broadcasting and EntertainmentOccupationActressYears active2001-presentSpouse(m. 2010)Korean nameHangul최자혜Hanja崔慈惠Revised RomanizationChoe Ja-hyeMcCune–ReischauerCh'oe Cha-hye Choi Ja-hye (born July 26, 1981) is a South Korean actress. She is best known for starring in the television drama King of...

هذه مقالة غير مراجعة. ينبغي أن يزال هذا القالب بعد أن يراجعها محرر مغاير للذي أنشأها؛ إذا لزم الأمر فيجب أن توسم المقالة بقوالب الصيانة المناسبة. يمكن أيضاً تقديم طلب لمراجعة المقالة في الصفحة المخصصة لذلك. (نوفمبر 2020) الجامعة التونسية للريشة الطائرة الاسم المختصر FTBAD الريا...

Luxol StadiumLocation Pembroke, MaltaCoordinates35°55′26″N 14°28′31″E / 35.92389°N 14.47528°E / 35.92389; 14.47528OwnerSt. Andrews F.C.OperatorSt. Andrews F.C.Capacity800SurfaceArtificial TurfOpenedMay 2006 (Artificial Turf)TenantsSt. Lucia F.C. St. Andrews F.C. (Not in the Maltese Premier League)Maltese National Amateur LeagueWebsitehttps://www.luxolsportsclub.com/ The Luxol Stadium is a stadium in Pembroke, Malta, opened on 26 May 2006. It was built at a ...

Estrellas Del Aire IATA ICAO Callsign - ETA[1] ESTRELLAS[2] Founded1991Commenced operationsSeptember 1991Ceased operationsNovember 1996Operating basesLic. Benito Juarez International AirportFleet size2Parent companyLínea Estrella de OroEmployees47 (in 1995)[3] Estrellas del Aire (English: Air Stars) was a Mexican passenger airline. It was established in 1991 by Estrellas de Oro, to complement their existing bus routes throughout Mexico. It was notable for being one of...

American politician James Craig TaylorAttorney General of VirginiaIn officeJanuary 19, 1870 – January 1, 1874GovernorGilbert Carlton WalkerPreceded byCharles WhittleseySucceeded byRaleigh Travers DanielMember of the Virginia Senatefrom the Carroll, Floyd, Grayson, Montgomery and Pulaski Counties districtIn officeSeptember 7, 1863 – March 15, 1865Preceded byJohn DickensonSucceeded byn/aMember of the Virginia House of Delegatesfrom the Montgomery districtIn off...

Barony in the Peerage of England Arms of Lucas of Little Saxham, Suffolk and Shenfield, Essex: Argent, a fess between six annulets gules Baron Lucas is a title that has been created twice in the Peerage of England. The second creation is extant and is currently held with the title Lord Dingwall in the Peerage of Scotland. Barons Lucas (of Shenfield) (1645) The title Baron Lucas, of Shenfield in the County of Essex, was created 13 January 1645 for Sir John Lucas, a Royalist army officer.[1...