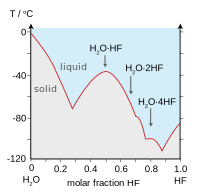
Hydrogen fluoride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

BangsriDesaKantor Desa BangsriNegara IndonesiaProvinsiJawa TengahKabupatenKaranganyarKecamatanKarangpandanKode pos57791Kode Kemendagri33.13.08.2001 Luas4,17 km²Jumlah penduduk... jiwaKepadatan663 jiwa/km² Bangsri adalah desa di kecamatan Karangpandan, Karanganyar, Jawa Tengah, Indonesia. Pembagian wilayah Desa Bangsri terdiri dari dukuh: Bendungan Bangsri Koncang Kopenan Ngumpeng Jangganan Kejenan Tangkilan Purwoasri Ngringin Selorejo Gondangrejo Toyo Tambong Pengkol Depok Ngipik lbsKe...

Divers at Work on the Wreck of the MaineA frame from the filmSutradara Georges Méliès ProduserDitulis olehTanggal rilis1898DurasiPendekNegara Prancis BahasaFilm bisu Visite sous-marine du Maine, yang dirilis di Amerika Serikat dengan judul Divers at Work on the Wreck of the Maine dan di Britania Raya dengan judul Divers at Work on a Wreck Under Sea,[1] adalah sebuah film bisu pendek Prancis tahun 1898 garapan Georges Méliès. Film tersebut dirilis oleh Star Film Company milik Méli...

Sporting event delegationBulgaria at the2023 World Aquatics ChampionshipsFlag of BulgariaFINA codeBULNational federationBulgarian Swimming FederationWebsitebul-swimming.orgin Fukuoka, JapanCompetitors6 in 2 sportsMedals Gold 0 Silver 0 Bronze 0 Total 0 World Aquatics Championships appearances197319751978198219861991199419982001200320052007200920112013201520172019202220232024 Bulgaria is set to compete at the 2023 World Aquatics Championships in Fukuoka, Japan from 14 to 30 July. Artistic swim...

This article is about local government in New South Wales. For a list of local government areas, see Local government areas of New South Wales. Politics of New South Wales Government Governor Premier Deputy Premier Treasurer Attorney General Leader of the Opposition Executive Council Current cabinet Parliament Council members Assembly members districts Judiciary Supreme Court District Court Local Court Political parties Animal Justice Greens Labor Liberals Nationals One Nation Shooters, Fishe...

Kladky Símbolos Bandeira Brasão de armas Localização KladkyLocalização de Kladky na Chéquia Coordenadas 49° 38' 57 N 16° 49' 42 E País Chéquia Região Olomouc Distrito Prostějov Características geográficas Área total 13,12 km² População total (2011) 389 hab. Densidade 29,6 hab./km² Altitude 505 m Fuso horário +1 Código Postal 798 54 Código estatístico 589594 Placa PV Sítio site Kladky é uma comuna checa localizada na região de Olomouc, ...

British-American broadcaster, filmmaker, author Roger BennettBennett in 2022BornRoger James Bennett (1970-09-14) 14 September 1970 (age 53)Liverpool, United KingdomOther namesRog BennettCitizenshipUnited StatesOccupation(s)Journalist, author, radio presenter, film makerKnown forMen in BlazersSpouse Vanessa Kroll (m. 2000)[1]RelativesNick Kroll (brother-in-law) Roger James Bennett (born 14 September 1970) is a British-American broadcaster, ...

لمعانٍ أخرى، طالع بلدة غرينوود (توضيح). بلدة غرينوود الإحداثيات 44°47′33″N 84°18′41″W / 44.7925°N 84.311388888889°W / 44.7925; -84.311388888889 [1] تقسيم إداري البلد الولايات المتحدة التقسيم الأعلى مقاطعة أوسكودا خصائص جغرافية المساحة 70.8 ميل مربع ارتفاع 359

Hedda HopperHedda Hopper pada 1930LahirElda Furry(1885-05-02)2 Mei 1885Hollidaysburg, Pennsylvania, ASMeninggal1 Februari 1966(1966-02-01) (umur 80)Hollywood, California, ASSebab meninggalPneumonia gandaMakamRose Hill Cemetery di Altoona, PennsylvaniaKebangsaanAmerikaPekerjaanAktris, kolumnis gosipTahun aktif1908–1966Dikenal atasWriting Hedda Hopper's HollywoodPartai politikRepublikSuami/istriDeWolf Hopper (m. 1913; bercerai 1922)&...

Mexican politician In this Spanish name, the first or paternal surname is Jiménez and the second or maternal family name is Salinas. Manolo Jiménez SalinasJiménez in 2023Governor of CoahuilaIncumbentAssumed office 1 December 2023Preceded byMiguel Riquelme Solís Personal detailsBorn (1984-06-12) 12 June 1984 (age 39)Saltillo, CoahuilaPolitical partyInstitutional Revolutionary PartySpousePaola Rodríguez López[1]Children4OccupationPolitician Manolo Jiménez Salinas...

17th century Holy Roman Empress For the Empress of Brazil, see Maria Leopoldina of Austria. Maria Leopoldine of AustriaPortrait by Lorenzo Lippi, 1649Holy Roman Empress (more...) Tenure2 July 1648 – 7 August 1649Born(1632-04-06)6 April 1632Innsbruck, TyrolDied7 August 1649(1649-08-07) (aged 17)Vienna, AustriaBurialImperial Crypt, Vienna, AustriaSpouse Ferdinand III, Holy Roman Emperor (m. 1648)IssueArchduke Charles Joseph of AustriaHouseHabsburgFa...

Association football club in England Football clubNostell Miners WelfareFull nameNostell Miners Welfare Football ClubNickname(s)The WelfareFounded1928GroundWelfare GroundCrofton, West YorkshireCapacity1,000 100 seats 100 covered standing [1]ChairmanKevin AllsopManagerIan WalkerLeagueNorthern Counties East League Division One2022–23Northern Counties East League Division One, 12th of 20 Home colours Away colours Nostell Miners Welfare Football Club is an English football club based in...

20023 film by Rafal Zielinski Hangman's CurseDirected byRafal ZielinskiScreenplay by Kathy Mackel Stan Foster Based onHangman's Curseby Frank PerettiProduced by Jerry Rose Rich Cowan Ralph Winter Joe Goodman Bobby Neutz Frank Peretti Steve Buhai Kelly Neutz Starring David Keith Mel Harris Leighton Meester Douglas Smith Frank Peretti Daniel Farber William R. Moses CinematographyDan HeighEdited by Mary Morrisey Tiffany Wallach Music byDavid BergeaudProductioncompanies Total Living Network Names...

Town in Arkansas, United StatesPleasant Plains, ArkansasTownLocation of Pleasant Plains in Independence County, ArkansasCoordinates: 35°33′16″N 91°37′38″W / 35.55444°N 91.62722°W / 35.55444; -91.62722CountryUnited StatesStateArkansasCountyIndependenceArea[1] • Total1.01 sq mi (2.61 km2) • Land1.01 sq mi (2.61 km2) • Water0.00 sq mi (0.00 km2)Elevation[2]597 ...

American role-playing game publisher Necromancer GamesIndustryRole-playing gameFounded2000Defunctc. 2010FateCurrently activeHeadquartersSeattle, WashingtonKey peopleClark Peterson and Bill Webb (founders)Productsd20 system licensed line Necromancer Games was an American publisher of role-playing games. With offices in Seattle, Washington and Coeur d'Alene, Idaho, the company specialized in material for the d20 System. Most of its products were released under the Open Game License of Wiz...

Autogrammkarte, Fotograf O. Schäffler, Heilbronn Wolf Ackva (* 30. Juli 1911 in Montigny-lès-Metz, Reichsland Elsaß-Lothringen/Deutsches Kaiserreich, heute Frankreich; † 16. Januar 2000 in Viehbach, Bayern[1]) war ein deutscher Schauspieler und Synchronsprecher. Inhaltsverzeichnis 1 Leben 2 Theaterrollen (Auswahl) 3 Filmografie (Auswahl) 4 Hörspiele (Auswahl) 5 Literatur 6 Weblinks 7 Einzelnachweise Leben Ackva arbeitete zunächst in Berlin als Autor des Mosse-Verlags. 1931...

U.S. highway in South Carolina This article is about the section of U.S. Route 278 in South Carolina. For the entire route, see U.S. Route 278. U.S. Highway 278Route of US 278 in South Carolina highlighted in redRoute informationAuxiliary route of US 78Maintained by SCDOTLength146.130 mi[1][2][3][4][5][6] (235.173 km)Existed1965[citation needed]–presentTouristroutes Hilton Head Scenic BywayMajor junctionsWest e...

1988 single by Bros Drop the BoySingle by Brosfrom the album Push B-sideThe Boy Is DroppedReleased7 March 1988 (1988-03-07)[1]Length3:50LabelCBSSongwriter(s)Nicky Graham, Tom WatkinsProducer(s)Nicky GrahamBros singles chronology When Will I Be Famous? (1987) Drop the Boy (1988) I Owe You Nothing (1988) Music videoDrop The Boy on YouTube Professional ratingsReview scoresSourceRatingNumber One[2] Drop the Boy is a song by British boy band Bros. It was written by N...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada November 2022. Hervé Bugnet Informasi pribadiNama lengkap Hervé BugnetTanggal lahir 24 Agustus 1981 (umur 42)Tempat lahir Sainte-Foy-la-Grande, PrancisTinggi 1,83 m (6 ft 0 in)Posisi bermain PenyerangInformasi klubKlub saat ini Évian TGFCNomor...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Skewb Ultimate – news · newspapers · books · scholar · JSTOR (April 2019) (Learn how and when to remove this template message) Meffert's version of the 6-color Skewb Ultimate The Skewb Ultimate, originally marketed as the Pyraminx Ball, is a twelve-sided puzzle derivation of the...

Sect of shaivism This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Veerashaiva – news · newspapers · books · scholar · JSTOR (June 2021) (Learn how and when to remove this template message) Part of a series onShaivism DeitiesParamashiva(Supreme being) Shiva Sadasiva Bhairava Rudra Virabhadra Shakti Parvati Sat...