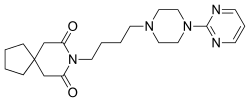
Buspiron
Buspiron (Buspar ) je anksiolitički psihoaktivni lek iz azapironske hemijske klase. On se prvenstveno koristi za tretiranje generalizovanog anksioznog poremećaja .
Medicinska upotreba
Hemija
Sinteza buspirona počinje sa N-alkilacijom 1-(2-pirimidil)piperazina sa 4-hlorobutironitrilom čemu sledi hidrogenacija nitrila preko Ranejev nikal katalizatora. Primarni aminski produkt prethodnog koraka sa spirocikličnim kiselim anhidridom daje buspiron.[ 10]
Reference
↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.” . Drug Discov Today 15 (23-24): 1052-7. DOI :10.1016/j.drudis.2010.10.003 . PMID 20970519 . edit ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4 : 217-241. DOI :10.1016/S1574-1400(08)00012-1 . ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining” . J Cheminform 2 (1): 3. DOI :10.1186/1758-2946-2-3 . PMID 20331846 . edit ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG” . Yeast 17 (1): 48–55. DOI :10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H . ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI :10.1093/nar/gkr777 . PMID 21948594 . edit ↑ „Buspirone monograph” . Drugs.com. Pristupljeno 27. 8. 2011 . ↑ National Institute Of Health. „Questions and Answers about the NIMH Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study — All Medication Levels” . Arhivirano iz originala na datum 2012-08-19. Pristupljeno 12. 8. 2012 . ↑ Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ (March 2006). „Medication augmentation after the failure of SSRIs for depression”. N. Engl. J. Med. 354 (12): 1243–52. DOI :10.1056/NEJMoa052964 . PMID 16554526 . ↑ Appelberg BG, Syvälahti EK, Koskinen TE, Mehtonen OP, Muhonen TT, Naukkarinen HH (June 2001). „Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors and in a Class of Drugs called Benzodiazepines and effects similar if not idenical to Alprazelam,Lorazepam etc : results from a placebo-controlled, randomized, double-blind, placebo wash-in study”. J Clin Psychiatry 62 (6): 448–52. PMID 11465522 . ↑ Wu YH, Rayburn JW, Allen LE, Ferguson HC, Kissel JW (May 1972). „Psychosedative agents. 2. 8-(4-Substituted 1-piperazinylalkyl)-8-azaspiro(4.5)decane-7,9-diones”. J. Med. Chem. 15 (5): 477–9. DOI :10.1021/jm00275a009 . PMID 5035267 . DE 2057845 , Šablon:Cite patent/authors , "Heterocyclische Azaspirodecandione und Verfahren zu ihrer Herstellung", published 9. 6. 1971. US 3717634 , Šablon:Cite patent/authors , "N-(heteroarcyclic)piperazinylalkyl-azaspiroalkanediones", published 20. 2. 1973. US 3907801 , Šablon:Cite patent/authors , "N8 (4-pyridyl-piperazino)-alkyl9-azaspiroalkanediones", published 23. 9. 1975. US 3976776 , Šablon:Cite patent/authors , "Tranquilizer process employing N-(heteroarcyclic)piperazinylalkylazaspiroalkanediones", published 24. 8. 1976.
Povezano
Agonisti :
5-FNE •
6-FNE •
Amidefrin •
Anisodamin •
Anisodin •
Cirazolin •
Dipivefrin •
Dopamin •
Efedrin •
Epinefrin (Adrenalin) •
Etilefrin •
Etilnorepinefrin •
Indanidin •
Levonordefrin •
Metaraminol •
Metoksamin •
Metildopa •
Midodrin •
Nafazolin •
Norepinefrin (Noradrenalin) •
Oktopamin •
Oksimetazolin •
Fenilefrin •
Fenilpropanolamin •
Pseudoefedrin •
Sinefrin •
Tetrahidrozolin Antagonisti :
Abanohil •
Adimolol •
Ajmalicin •
Alfuzosin •
Amosulalol •
Arotinolol •
Atiprosin •
Benoksatian •
Buflomedil •
Bunazosin •
Karvedilol •
CI-926 •
Korinantin •
Dapiprazol •
DL-017 •
Domesticin •
Doksazosin •
Eugenodilol •
Fenspirid •
GYKI-12,743 •
GYKI-16,084 •
Indoramin •
Ketanserin •
L-765,314 •
Labetalol •
Mefendioksan •
Metazosin •
Monatepil •
Moksisilit (Timoksamin) •
Naftopidil •
Nantenin •
Neldazosin •
Nicergolin •
Niguldipin •
Pelanserin •
Fendioksan •
Fenoksibenzamin •
Fentolamin •
Piperoksan •
Prazosin •
Hinazosin •
Ritanserin •
RS-97,078 •
SGB-1,534 •
Silodosin •
SL-89.0591 •
Spiperon •
Talipeksol •
Tamsulosin •
Terazosin •
Tibalosin •
Tiodazosin •
Tipentosin •
Tolazolin •
Trimazosin •
Upidosin •
Urapidil •
Zolertin * Mnogi TCA , TeCA , antipsihotici , ergolini , i neki piperazini kao što su buspiron , trazodon , nefazodon , etoperidon , i mepiprazol takođe antagoniziraju α1 -adrenergičke receptore, što doprinosi njihovim nuspojavama.
Adamantani :
Amantadin •
Memantin •
Rimantadin ;
Aminotetralini :
7-OH-DPAT •
8-OH-PBZI •
Rotigotin •
UH-232 ;
Benzazepini :
6-Br-APB •
Fenoldopam •
SKF-38,393 •
SKF-77,434 •
SKF-81,297 •
SKF-82,958 •
SKF-83,959 ;
Ergolini :
Bromokriptin •
Kabergolin •
Dihidroergokriptin •
Lisurid •
LSD •
Pergolid ;
Dihidreksidinski derivati :
2-OH-NPA •
A-86,929 •
Ciladopa •
Dihidreksidin •
Dinapsolin •
Dinoksilin •
Doksantrin ;
Drugi :
A-68,930 •
A-77636 •
A-412,997 •
ABT-670 •
ABT-724 •
Aplindor •
Apomorfin •
Aripiprazol •
Bifeprunoks •
BP-897 •
CY-208,243 •
Dizocilpin •
Etilevodopa •
Flibanserin •
Ketamin •
Melevodopa •
Modafinil •
Pardoprunoks •
Fenciklidin •
PD-128,907 •
PD-168,077 •
PF-219,061 •
Piribedil •
Pramipeksol •
Propilnorapomorfin •
Pukatein •
Hinagolid •
Hineloran •
Hinpirol •
RDS-127 •
Ro10-5824 •
Ropinirol •
Rotigotin •
Roksindol •
Salvinorin A •
SKF-89,145 •
Sumanirol •
Tergurid •
Umespiron •
WAY-100,635
Morfolini :
Fenbutrazat •
Morazon •
Fendimetrazin •
Fenmetrazin ;
Oksazolini :
4-Metilaminoreks (4-MAR, 4-MAX) •
Aminoreks •
Klominoreks •
Ciklazodon •
Fenozolon •
Fluminoreks •
Pemolin •
Tozalinon ;
Fenetilamini (takođe
amfetamini ,
katinoni ,
fentermini , itd):
2-Hidroksifenetilamin (2-OH-PEA) •
4-CAB •
4-Metilamfetamin (4-MA) •
4-Metilmetamfetamin (4-MMA) •
Alfetamin •
Amfekloral •
Amfepentoreks •
Amfepramon •
Amfetamin (
Dekstroamfetamin •
Levoamfetamin ) •
Amfetaminil •
β-Metilfenetilamin (β-Me-PEA) •
Benzodioksolilbutanamin (BDB) •
Benzodioksolilhidroksibutanamin (BOH) •
Benzfetamin •
Bufedron •
Butilon •
Katin •
Katinon •
Klobenzoreks •
Klortermin •
D-deprenil •
Dimetoksiamfetamin (DMA) •
Dimetoksimetamfetamin (DMMA) •
Dimetilamfetamin •
Dimetilkatinon (Dimetilpropion, metamfepramon) •
Etkatinon (Etilpropion) •
Etilamfetamin •
Etilbenzodioksolilbutanamin (EBDB) •
Etilon •
Famprofazon •
Fenetilin •
Fenproporeks •
Flefedron •
Fludoreks •
Furfenoreks •
Hordenin •
Lofofin (Homomiristicilamin) •
Mefenoreks •
Mefedron •
Metamfetamin (Dezoksiefedrin, Metedrin;
Dekstrometamfetamin •
Levometamfetamin ) •
Metkatinon (Metilpropion) •
Metedron •
Metoksimetilenedioksiamfetamin (MMDA) •
Metoksimetilendioksimetamfetamin (MMDMA) •
Metilbenzodioksolilbutanamin (MBDB) •
Metilendioksiamfetamin (MDA, tenamfetamin) •
Metilendioksietilamfetamin (MDEA) •
Metilendioksihidroksiamfetamin (MDOH) •
Metilendioksimetamfetamin (MDMA) •
Metilendioksimetilfenetilamin (MDMPEA, homarilamin) •
Metilendioksifenetilamin (MDPEA, homopiperonilamin) •
Metilon •
Ortetamin •
Parabromoamfetamin (PBA) •
Parahloroamfetamin (PCA) •
Parafluoroamfetamin (PFA) •
Parafluorometamfetamin (PFMA) •
Parahidroksiamfetamin (PHA) •
Parajodoamfetamin (PIA) •
Paredrin (Norfoledrin, Oksamfetamin) •
Fenetilamin (PEA) •
Foledrin •
Fenprometamin •
Prenilamin •
Propilamfetamin •
Tifloreks (Flutioreks) •
Tiramin (TRA) •
Ksilopropamin •
Zilofuramin ;
Piperazini :
2,5-Dimetoksi-4-bromobenzilpiperazin (2C-B-BZP) •
Benzilpiperazin (BZP) •
Metoksifenilpiperazin (MeOPP, paraperazin) •
Metilbenzilpiperazin (MBZP) •
Metilendioksibenzilpiperazin (MDBZP, piperonilpiperazin);
Drugi :
2-Amino-1,2-dihidronaftalen (2-ADN) •
2-Aminoindan (2-AI) •
2-Aminotetralin (2-AT) •
4-Benzilpiperidin (4-BP) •
5-IAI •
Klofenciklan •
Ciklopentamin •
Cipenamin •
Ciprodenat •
Feprosidnin •
Gilutensin •
Heptaminol •
Heksaciklonat •
Indanilaminopropan (IAP) •
Indanoreks •
Izometepten •
Metilheksanamin •
Naftilaminopropan (NAP) •
Oktodrin •
Ftalimidopropiofenon •
Propilheksedrin (
Levopropilheksedrin ) •
Tuaminoheptan (Tuamin)
Agonisti :
Azapironi :
Alnespiron •
Binospiron •
Buspiron •
Enilospiron •
Eptapiron •
Gepiron •
Ipsapiron •
Perospiron •
Revospiron •
Tandospiron •
Tiospiron •
Umespiron •
Zalospiron ;
Antidepresivi :
Etoperidon •
Nefazodon •
Trazodon ;
Antipsihotici :
Aripiprazol •
Asenapin •
Klozapin •
Kvetiapin •
Ziprasidon ;
Ergolini :
Dihidroergotamin •
Ergotamin •
Lisurid •
Metisergid •
LSD ;
Triptamini :
5-CT •
5-MeO-DMT •
5-MT •
Bufotenin •
DMT •
Indorenat •
Psilocin •
Psilocibin ;
Drugi :
8-OH-DPAT •
Adatanserin •
Bay R 1531 •
Befiradol •
BMY-14802 •
Kanabidiol •
Dimemebfe •
Ebalzotan •
Eltoprazin •
F-11,461 •
F-12,826 •
F-13,714 •
F-14,679 •
F-15,063 •
F-15,599 •
Flesinoksan •
Flibanserin •
Lesopitron •
Lu AA21004 •
LY-293,284 •
LY-301,317 •
MKC-242 •
NBUMP •
Osemozotan •
Oksaflozan •
Pardoprunoks •
Piklozotan •
Rauvolscin •
Repinotan •
Roksindol •
RU-24,969 •
S 14,506 •
S-14,671 •
S-15,535 •
Sarizotan •
SSR-181,507 •
Sunepitron •
U-92,016-A •
Urapidil •
Vilazodon •
Ksaliproden •
Johimbin Antagonisti :
Antipsihotici :
Iloperidon •
Risperidon •
Sertindol ;
Beta blokatori :
Alprenolol •
Cianopindolol •
Jodocianopindolol •
Oksprenolol •
Pindobind •
Pindolol •
Propranolol •
Tertatolol ;
Drugi :
AV965 •
BMY-7,378 •
CSP-2503 •
Dotarizin •
Flopropion •
GR-46611 •
Isamoltan •
Lekozotan •
Metitepin/Metiotepin •
MPPF •
NAN-190 •
PRX-00023 •
Robalzotan •
S-15535 •
SB-649,915 •
SDZ 216-525 •
Spiperon •
Spiramid •
Spiroksatrin •
UH-301 •
WAY-100,135 •
WAY-100,635 •
Ksilamidin Agonisti :
Lisergamidi :
Dihidroergotamin •
Metisergid ;
Triptani :
Almotriptan •
Avitriptan •
Eletriptan •
Frovatriptan •
Naratriptan •
Rizatriptan •
Sumatriptan •
Zolmitriptan ;
Triptamini :
5-CT •
5-Etil-DMT •
5-MT •
5-(Noniloksi)triptamin ;
Drugi :
CP-135,807 •
CP-286,601 •
GR-46611 •
L-694,247 •
L-772,405 •
PNU-109,291 •
PNU-142,633 Antagonisti :
Lisergamidi :
Metergolin ;
Drugi :
Alniditan •
BRL-15,572 •
Elzasonan •
GR-127,935 •
Ketanserin •
LY-310,762 •
LY-367,642 •
LY-456,219 •
LY-456,220 •
Metitepin/Metiotepin •
Ritanserin •
Johimbin •
Ziprasidon
Agonisti :
Fenetilamini :
2C-B •
2C-E •
2C-I •
2C-T-2 •
2C-T-7 •
2C-T-21 •
DOB •
DOC •
DOI •
DOM •
MDA •
MDMA •
Meskalin ;
Piperazini :
Aripiprazol •
mCPP •
TFMPP ;
Triptamini :
5-CT •
5-MeO-α-ET •
5-MeO-α-MT •
5-MeO-DET •
5-MeO-DiPT •
5-MeO-DMT •
5-MeO-DPT •
5-MT •
α-ET •
α-Metil-5-HT •
α-MT •
Bufotenin •
DET •
DiPT •
DMT •
DPT •
Psilocin •
Psilocibin ;
Drugi :
A-372,159 •
AL-38022A •
CP-809,101 •
Dimemebfe •
Lorkaserin •
Medifoksamin •
MK-212 •
Org 12,962 •
ORG-37,684 •
Oksaflozan •
PNU-22394 •
Ro60-0175 •
Ro60-0213 •
Vabicaserin •
WAY-629 •
WAY-161,503 •
YM-348 Antagonisti :
Atipični antipsihotici :
Klozapin •
Iloperidon •
Melperon •
Olanzapin •
Paliperidon •
Pimozid •
Hetiapin •
Risperidon •
Sertindol •
Ziprasidon •
Zotepin ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin •
Pipamperon ;
Antidepresivi :
Agomelatin •
Amitriptilin •
Amoksapin •
Aptazapin •
Etoperidon •
Fluoksetin •
Mianserin •
Mirtazapin •
Nefazodon •
Nortriptilin •
Trazodon ;
Drugi :
Adatanserin •
Cinanserin •
Ciproheptadin •
Deramciklan •
Dotarizin •
Eltoprazin •
Esmirtazapin •
FR-260,010 •
Ketanserin •
Ketotifen •
Latrepirdin •
Lu AA24530 •
Metitepin/Metiotepin •
Metisergid •
Pizotifen •
Ritanserin •
RS-102,221 •
S-14,671 •
SB-200,646 •
SB-206,553 •
SB-221,284 •
SB-228,357 •
SB-242,084 •
SB-243,213 •
SDZ SER-082 •
Ksilamidin
Agonisti :
Lisergamidi :
Dihidroergotamin •
Ergotamin •
Lisurid •
LSD •
Mesulergin •
Metergolin •
Metisergid ;
Triptamini :
2-Metil-5-HT •
5-BT •
5-CT •
5-MT •
Bufotenin •
E-6801 •
E-6837 •
EMD-386,088 •
EMDT •
LY-586,713 •
N -Metil-5-HT •
Triptamin ;
Drugi :
WAY-181,187 •
WAY-208,466 Antagonisti :
Antidepresanti :
Amitriptilin •
Amoksapin •
Klomipramin •
Doksepin •
Mianserin •
Nortriptilin ;
Atipični antipsihotici :
Aripiprazol •
Asenapin •
Klozapin •
Fluperlapin •
Iloperidon •
Olanzapin •
Tiospiron ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin ;
Drugi :
BGC20-760 •
BVT-5182 •
BVT-74316 •
EGIS-12,233 •
GW-742,457 •
Ketanserin •
Latrepirdin •
Lu AE58054 •
Metitepin/Metiotepin •
MS-245 •
PRX-07034 •
Ritanserin •
Ro04-6790 •
Ro 63-0563 •
SB-258,585 •
SB-271,046 •
SB-357,134 •
SB-399,885 •
SB-742,457 Agonisti :
Lisergamidi :
LSD ;
Triptamini :
5-CT •
5-MT •
Bufotenin ;
Drugi :
8-OH-DPAT •
AS-19 •
Bifeprunoks •
LP-12 •
LP-44 •
RU-24,969 •
Sarizotan Antagonisti :
Lisergamidi :
2-Bromo-LSD •
Bromokriptin •
Dihidroergotamin •
Ergotamin •
Mesulergin •
Metergolin •
Metisergid ;
Antidepresanti :
Amitriptilin •
Amoksapin •
Klomipramin •
Imipramin •
Maprotilin •
Mianserin ;
Atipični antipsihotici :
Amisulprid •
Aripiprazol •
Klozapin •
Olanzapin •
Risperidon •
Sertindol •
Tiospiron •
Ziprasidon •
Zotepin ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin ;
Drugi :
Butaklamol •
EGIS-12,233 •
Ketanserin •
LY-215,840 •
Metitepin/Metiotepin •
Pimozid •
Ritanserin •
SB-258,719 •
SB-258,741 •
SB-269,970 •
SB-656,104 •
SB-656,104-A •
SB-691,673 •
SLV-313 •
SLV-314 •
Spiperon •
SSR-181,507