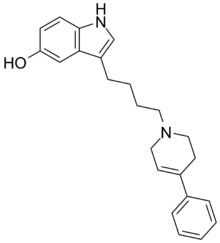
Roksindol
Roksindol
(IUPAC ) ime
3-[4-(4-fenil-3,6-dihidro-2H -piridin-1-il)butil]-1H -indol-5-ol
Klinički podaci
Identifikatori
CAS broj
112192-04-8
ATC kod
nije dodeljen
PubChem [ 1] [ 2] 219050
ChemSpider [ 3] 189880
UNII
43227SMS0O Y
Hemijski podaci
Formula
C 23 H 26 N 2 O
Mol. masa
346,47 g/mol
SMILES
eMolekuli PubHem
InChI InChI=1S/C23H26N2O/c26-21-9-10-23-22(16-21)20(17-24-23)8-4-5-13-25-14-11-19(12-15-25)18-6-2-1-3-7-18/h1-3,6-7,9-11,16-17,24,26H,4-5,8,12-15H2 Y Y
Farmakoinformacioni podaci
Trudnoća
?
Pravni status
Roksindol (EMD-49,980 ) je dopaminergični i serotonergični lek koji je originalno bio razvijen za lečenje šizofrenije .[ 4] [ 5] [ 6] kliničkim ispitivanjima njegova antipsihotička efikasnost je bila skromna, ali je neočekivano nađeno da proizvodi potentne i brze antidepresantne i anksiolitičke efekte.[ 5] [ 6] depresije .[ 4] [ 7] [ 7] Parkinsonovu bolest i lečenje prolaktinoma .[ 8] [ 9]
Roksindol deluje kao agonist na sledećim receptorima :[ 10] [ 11]
Reference
↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.” . Drug Discov Today 15 (23-24): 1052-7. DOI :10.1016/j.drudis.2010.10.003 . PMID 20970519 . edit ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4 : 217-241. DOI :10.1016/S1574-1400(08)00012-1 . ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining” . J Cheminform 2 (1): 3. DOI :10.1186/1758-2946-2-3 . PMID 20331846 . edit ↑ 4,0 4,1 Gründer G, Wetzel H, Hammes E, Benkert O (1993). „Roxindole, a dopamine autoreceptor agonist, in the treatment of major depression” . Psychopharmacology 111 (1): 123–6. DOI :10.1007/BF02257418 . PMID 7870927 . ↑ 5,0 5,1 Klimke A, Klieser E (July 1991). „Antipsychotic efficacy of the dopaminergic autoreceptor agonist EMD 49980 (Roxindol). Results of an open clinical study”. Pharmacopsychiatry 24 (4): 107–12. DOI :10.1055/s-2007-1014451 . PMID 1684439 . ↑ 6,0 6,1 Kasper S, Fuger J, Zinner HJ, Bäuml J, Möller HJ (March 1992). „Early clinical results with the neuroleptic roxindole (EMD 49,980) in the treatment of schizophrenia--an open study” . European Neuropsychopharmacology : the Journal of the European College of Neuropsychopharmacology 2 (1): 91–5. DOI :10.1016/0924-977X(92)90041-6 . PMID 1353388 . ↑ 7,0 7,1 Maj J, Kolodziejczyk K, Rogóz Z, Skuza G (1996). „Roxindole, a potential antidepressant. I. Effect on the dopamine system”. Journal of Neural Transmission (Vienna, Austria : 1996) 103 (5): 627–41. PMID 8811507 . ↑ Bravi D, Davis TL, Mouradian MM, Chase TN (April 1993). „Treatment of Parkinson's disease with the partial dopamine agonist EMD 49980”. Movement Disorders : Official Journal of the Movement Disorder Society 8 (2): 195–7. DOI :10.1002/mds.870080214 . PMID 8097280 . ↑ Jaspers C, Benker G, Reinwein D (June 1994). „Treatment of prolactinoma patients with the new non-ergot dopamine agonist roxindol: first results”. The Clinical Investigator 72 (6): 451–6. PMID 7950157 . ↑ Newman-Tancredi A, Cussac D, Audinot V, Millan MJ (June 1999). „Actions of roxindole at recombinant human dopamine D2, D3 and D4 and serotonin 5-HT1A, 5-HT1B and 5-HT1D receptors” . Naunyn-Schmiedeberg's Archives of Pharmacology 359 (6): 447–53. PMID 10431754 . ↑ Heinrich T, Böttcher H, Bartoszyk GD, Greiner HE, Seyfried CA, Van Amsterdam C (September 2004). „Indolebutylamines as selective 5-HT(1A) agonists”. Journal of Medicinal Chemistry 47 (19): 4677–83. DOI :10.1021/jm040792y . PMID 15341483 .
Serotoninski modulatori i stimulatori (SMS)
Neselektivni
MAOA -Selektivni
MAOB -Selektivni
Adamantani :
Amantadin •
Memantin •
Rimantadin ;
Aminotetralini :
7-OH-DPAT •
8-OH-PBZI •
Rotigotin •
UH-232 ;
Benzazepini :
6-Br-APB •
Fenoldopam •
SKF-38,393 •
SKF-77,434 •
SKF-81,297 •
SKF-82,958 •
SKF-83,959 ;
Ergolini :
Bromokriptin •
Kabergolin •
Dihidroergokriptin •
Lisurid •
LSD •
Pergolid ;
Dihidreksidinski derivati :
2-OH-NPA •
A-86,929 •
Ciladopa •
Dihidreksidin •
Dinapsolin •
Dinoksilin •
Doksantrin ;
Drugi :
A-68,930 •
A-77636 •
A-412,997 •
ABT-670 •
ABT-724 •
Aplindor •
Apomorfin •
Aripiprazol •
Bifeprunoks •
BP-897 •
CY-208,243 •
Dizocilpin •
Etilevodopa •
Flibanserin •
Ketamin •
Melevodopa •
Modafinil •
Pardoprunoks •
Fenciklidin •
PD-128,907 •
PD-168,077 •
PF-219,061 •
Piribedil •
Pramipeksol •
Propilnorapomorfin •
Pukatein •
Hinagolid •
Hineloran •
Hinpirol •
RDS-127 •
Ro10-5824 •
Ropinirol •
Rotigotin •
Roksindol •
Salvinorin A •
SKF-89,145 •
Sumanirol •
Tergurid •
Umespiron •
WAY-100,635
Morfolini :
Fenbutrazat •
Morazon •
Fendimetrazin •
Fenmetrazin ;
Oksazolini :
4-Metilaminoreks (4-MAR, 4-MAX) •
Aminoreks •
Klominoreks •
Ciklazodon •
Fenozolon •
Fluminoreks •
Pemolin •
Tozalinon ;
Fenetilamini (takođe
amfetamini ,
katinoni ,
fentermini , itd):
2-Hidroksifenetilamin (2-OH-PEA) •
4-CAB •
4-Metilamfetamin (4-MA) •
4-Metilmetamfetamin (4-MMA) •
Alfetamin •
Amfekloral •
Amfepentoreks •
Amfepramon •
Amfetamin (
Dekstroamfetamin •
Levoamfetamin ) •
Amfetaminil •
β-Metilfenetilamin (β-Me-PEA) •
Benzodioksolilbutanamin (BDB) •
Benzodioksolilhidroksibutanamin (BOH) •
Benzfetamin •
Bufedron •
Butilon •
Katin •
Katinon •
Klobenzoreks •
Klortermin •
D-deprenil •
Dimetoksiamfetamin (DMA) •
Dimetoksimetamfetamin (DMMA) •
Dimetilamfetamin •
Dimetilkatinon (Dimetilpropion, metamfepramon) •
Etkatinon (Etilpropion) •
Etilamfetamin •
Etilbenzodioksolilbutanamin (EBDB) •
Etilon •
Famprofazon •
Fenetilin •
Fenproporeks •
Flefedron •
Fludoreks •
Furfenoreks •
Hordenin •
Lofofin (Homomiristicilamin) •
Mefenoreks •
Mefedron •
Metamfetamin (Dezoksiefedrin, Metedrin;
Dekstrometamfetamin •
Levometamfetamin ) •
Metkatinon (Metilpropion) •
Metedron •
Metoksimetilenedioksiamfetamin (MMDA) •
Metoksimetilendioksimetamfetamin (MMDMA) •
Metilbenzodioksolilbutanamin (MBDB) •
Metilendioksiamfetamin (MDA, tenamfetamin) •
Metilendioksietilamfetamin (MDEA) •
Metilendioksihidroksiamfetamin (MDOH) •
Metilendioksimetamfetamin (MDMA) •
Metilendioksimetilfenetilamin (MDMPEA, homarilamin) •
Metilendioksifenetilamin (MDPEA, homopiperonilamin) •
Metilon •
Ortetamin •
Parabromoamfetamin (PBA) •
Parahloroamfetamin (PCA) •
Parafluoroamfetamin (PFA) •
Parafluorometamfetamin (PFMA) •
Parahidroksiamfetamin (PHA) •
Parajodoamfetamin (PIA) •
Paredrin (Norfoledrin, Oksamfetamin) •
Fenetilamin (PEA) •
Foledrin •
Fenprometamin •
Prenilamin •
Propilamfetamin •
Tifloreks (Flutioreks) •
Tiramin (TRA) •
Ksilopropamin •
Zilofuramin ;
Piperazini :
2,5-Dimetoksi-4-bromobenzilpiperazin (2C-B-BZP) •
Benzilpiperazin (BZP) •
Metoksifenilpiperazin (MeOPP, paraperazin) •
Metilbenzilpiperazin (MBZP) •
Metilendioksibenzilpiperazin (MDBZP, piperonilpiperazin);
Drugi :
2-Amino-1,2-dihidronaftalen (2-ADN) •
2-Aminoindan (2-AI) •
2-Aminotetralin (2-AT) •
4-Benzilpiperidin (4-BP) •
5-IAI •
Klofenciklan •
Ciklopentamin •
Cipenamin •
Ciprodenat •
Feprosidnin •
Gilutensin •
Heptaminol •
Heksaciklonat •
Indanilaminopropan (IAP) •
Indanoreks •
Izometepten •
Metilheksanamin •
Naftilaminopropan (NAP) •
Oktodrin •
Ftalimidopropiofenon •
Propilheksedrin (
Levopropilheksedrin ) •
Tuaminoheptan (Tuamin)
Agonisti :
Azapironi :
Alnespiron •
Binospiron •
Buspiron •
Enilospiron •
Eptapiron •
Gepiron •
Ipsapiron •
Perospiron •
Revospiron •
Tandospiron •
Tiospiron •
Umespiron •
Zalospiron ;
Antidepresivi :
Etoperidon •
Nefazodon •
Trazodon ;
Antipsihotici :
Aripiprazol •
Asenapin •
Klozapin •
Kvetiapin •
Ziprasidon ;
Ergolini :
Dihidroergotamin •
Ergotamin •
Lisurid •
Metisergid •
LSD ;
Triptamini :
5-CT •
5-MeO-DMT •
5-MT •
Bufotenin •
DMT •
Indorenat •
Psilocin •
Psilocibin ;
Drugi :
8-OH-DPAT •
Adatanserin •
Bay R 1531 •
Befiradol •
BMY-14802 •
Kanabidiol •
Dimemebfe •
Ebalzotan •
Eltoprazin •
F-11,461 •
F-12,826 •
F-13,714 •
F-14,679 •
F-15,063 •
F-15,599 •
Flesinoksan •
Flibanserin •
Lesopitron •
Lu AA21004 •
LY-293,284 •
LY-301,317 •
MKC-242 •
NBUMP •
Osemozotan •
Oksaflozan •
Pardoprunoks •
Piklozotan •
Rauvolscin •
Repinotan •
Roksindol •
RU-24,969 •
S 14,506 •
S-14,671 •
S-15,535 •
Sarizotan •
SSR-181,507 •
Sunepitron •
U-92,016-A •
Urapidil •
Vilazodon •
Ksaliproden •
Johimbin Antagonisti :
Antipsihotici :
Iloperidon •
Risperidon •
Sertindol ;
Beta blokatori :
Alprenolol •
Cianopindolol •
Jodocianopindolol •
Oksprenolol •
Pindobind •
Pindolol •
Propranolol •
Tertatolol ;
Drugi :
AV965 •
BMY-7,378 •
CSP-2503 •
Dotarizin •
Flopropion •
GR-46611 •
Isamoltan •
Lekozotan •
Metitepin/Metiotepin •
MPPF •
NAN-190 •
PRX-00023 •
Robalzotan •
S-15535 •
SB-649,915 •
SDZ 216-525 •
Spiperon •
Spiramid •
Spiroksatrin •
UH-301 •
WAY-100,135 •
WAY-100,635 •
Ksilamidin Agonisti :
Lisergamidi :
Dihidroergotamin •
Metisergid ;
Triptani :
Almotriptan •
Avitriptan •
Eletriptan •
Frovatriptan •
Naratriptan •
Rizatriptan •
Sumatriptan •
Zolmitriptan ;
Triptamini :
5-CT •
5-Etil-DMT •
5-MT •
5-(Noniloksi)triptamin ;
Drugi :
CP-135,807 •
CP-286,601 •
GR-46611 •
L-694,247 •
L-772,405 •
PNU-109,291 •
PNU-142,633 Antagonisti :
Lisergamidi :
Metergolin ;
Drugi :
Alniditan •
BRL-15,572 •
Elzasonan •
GR-127,935 •
Ketanserin •
LY-310,762 •
LY-367,642 •
LY-456,219 •
LY-456,220 •
Metitepin/Metiotepin •
Ritanserin •
Johimbin •
Ziprasidon
Agonisti :
Fenetilamini :
2C-B •
2C-E •
2C-I •
2C-T-2 •
2C-T-7 •
2C-T-21 •
DOB •
DOC •
DOI •
DOM •
MDA •
MDMA •
Meskalin ;
Piperazini :
Aripiprazol •
mCPP •
TFMPP ;
Triptamini :
5-CT •
5-MeO-α-ET •
5-MeO-α-MT •
5-MeO-DET •
5-MeO-DiPT •
5-MeO-DMT •
5-MeO-DPT •
5-MT •
α-ET •
α-Metil-5-HT •
α-MT •
Bufotenin •
DET •
DiPT •
DMT •
DPT •
Psilocin •
Psilocibin ;
Drugi :
A-372,159 •
AL-38022A •
CP-809,101 •
Dimemebfe •
Lorkaserin •
Medifoksamin •
MK-212 •
Org 12,962 •
ORG-37,684 •
Oksaflozan •
PNU-22394 •
Ro60-0175 •
Ro60-0213 •
Vabicaserin •
WAY-629 •
WAY-161,503 •
YM-348 Antagonisti :
Atipični antipsihotici :
Klozapin •
Iloperidon •
Melperon •
Olanzapin •
Paliperidon •
Pimozid •
Hetiapin •
Risperidon •
Sertindol •
Ziprasidon •
Zotepin ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin •
Pipamperon ;
Antidepresivi :
Agomelatin •
Amitriptilin •
Amoksapin •
Aptazapin •
Etoperidon •
Fluoksetin •
Mianserin •
Mirtazapin •
Nefazodon •
Nortriptilin •
Trazodon ;
Drugi :
Adatanserin •
Cinanserin •
Ciproheptadin •
Deramciklan •
Dotarizin •
Eltoprazin •
Esmirtazapin •
FR-260,010 •
Ketanserin •
Ketotifen •
Latrepirdin •
Lu AA24530 •
Metitepin/Metiotepin •
Metisergid •
Pizotifen •
Ritanserin •
RS-102,221 •
S-14,671 •
SB-200,646 •
SB-206,553 •
SB-221,284 •
SB-228,357 •
SB-242,084 •
SB-243,213 •
SDZ SER-082 •
Ksilamidin
Agonisti :
Lisergamidi :
Dihidroergotamin •
Ergotamin •
Lisurid •
LSD •
Mesulergin •
Metergolin •
Metisergid ;
Triptamini :
2-Metil-5-HT •
5-BT •
5-CT •
5-MT •
Bufotenin •
E-6801 •
E-6837 •
EMD-386,088 •
EMDT •
LY-586,713 •
N -Metil-5-HT •
Triptamin ;
Drugi :
WAY-181,187 •
WAY-208,466 Antagonisti :
Antidepresanti :
Amitriptilin •
Amoksapin •
Klomipramin •
Doksepin •
Mianserin •
Nortriptilin ;
Atipični antipsihotici :
Aripiprazol •
Asenapin •
Klozapin •
Fluperlapin •
Iloperidon •
Olanzapin •
Tiospiron ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin ;
Drugi :
BGC20-760 •
BVT-5182 •
BVT-74316 •
EGIS-12,233 •
GW-742,457 •
Ketanserin •
Latrepirdin •
Lu AE58054 •
Metitepin/Metiotepin •
MS-245 •
PRX-07034 •
Ritanserin •
Ro04-6790 •
Ro 63-0563 •
SB-258,585 •
SB-271,046 •
SB-357,134 •
SB-399,885 •
SB-742,457 Agonisti :
Lisergamidi :
LSD ;
Triptamini :
5-CT •
5-MT •
Bufotenin ;
Drugi :
8-OH-DPAT •
AS-19 •
Bifeprunoks •
LP-12 •
LP-44 •
RU-24,969 •
Sarizotan Antagonisti :
Lisergamidi :
2-Bromo-LSD •
Bromokriptin •
Dihidroergotamin •
Ergotamin •
Mesulergin •
Metergolin •
Metisergid ;
Antidepresanti :
Amitriptilin •
Amoksapin •
Klomipramin •
Imipramin •
Maprotilin •
Mianserin ;
Atipični antipsihotici :
Amisulprid •
Aripiprazol •
Klozapin •
Olanzapin •
Risperidon •
Sertindol •
Tiospiron •
Ziprasidon •
Zotepin ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin ;
Drugi :
Butaklamol •
EGIS-12,233 •
Ketanserin •
LY-215,840 •
Metitepin/Metiotepin •
Pimozid •
Ritanserin •
SB-258,719 •
SB-258,741 •
SB-269,970 •
SB-656,104 •
SB-656,104-A •
SB-691,673 •
SLV-313 •
SLV-314 •
Spiperon •
SSR-181,507